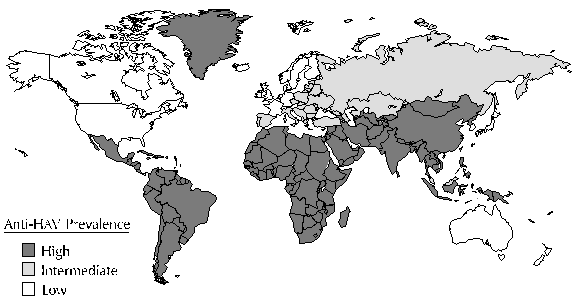
Risk:
The risk of hepatitis A for U.S. citizens traveling abroad varies with living conditions, length of
stay, and the incidence of HAV infection in areas visited. In general, travelers to northern
and western Europe, Japan, Australia, New Zealand and North America (except Mexico) are at
no greater risk of infection than they would be in the U.S. Areas of the world with intermediate
or high rates of hepatitis A do pose an increased risk for travelers (Figure 1). For travelers to
developing countries, risk of infection increases with duration of travel and is highest for those
who live in or visit rural areas, trek in back country, or frequently eat or drink in settings of poor
sanitation. Recent studies have shown that many cases of travel-related hepatitis A occur in
travelers with "standard" tourist itineraries, accommodations, and food and beverage consumption
behaviors. In developing countries, travelers should minimize their exposure to
hepatitis A and other enteric diseases by avoiding potentially contaminated water or
food. Travelers should avoid drinking water (or beverages with ice) of unknown purity and
eating uncooked shellfish or uncooked fruits or vegetables that are not peeled or prepared by the
traveler.
Recommendations:
Hepatitis A vaccine or immune globulin (IG) is recommended for all susceptible travelers to or for
persons working in countries with intermediate or high rates of HAV infection. Vaccination
of children 2 years of age and older, adolescents and adults with the age-appropriate dose of
hepatitis A vaccine is preferred for persons who plan to travel repeatedly or reside for long
periods in intermediate or high risk areas. Because of the current IG shortage, vaccine is also preferred for travelers 2 years of age and older desiring only short-term protection.
Immune globulin is recommended for travelers less than 2 years of age.
Dosing Information:
There are two hepatitis A vaccines currently licensed in the United States: HAVRIX®
(manufactured by SmithKline Beecham Biologicals), and VAQTA® (manufactured by Merck &
Co., Inc.). Both are inactivated vaccines, adsorbed to aluminum hydroxide as an adjuvant.
HAVRIX® contains 2-phenoxyethanol as a preservative.
The vaccine should be administered by intramuscular injection into the deltoid muscle. It is licensed in adult and pediatric formulations, with different dosages and administration schedules. For HAVRIX®, adults (> 18 years) should be given two 1440 Elisa Unit (EL.U.) doses with the second dose administered 6 to 12 months after the first dose. There are two dosage schedules for children and adolescents (ages 2-18): a) 2 doses of 720 EL.U.of HAVRIX® with the second dose administered 6 to 12 months after the first dose, and b) 3 doses of 360 EL.U. of HAVRIX® with the second dose administered one month after the first dose and the third dose administered 6 to 12 months after the first dose. For VAQTA®, adults (> 17 years) should be given two 50 unit (U) doses with the second dose administered 6 months later. The schedule for children and adolescents (ages 2-17) includes 2 doses of VAQTA® with the second dose administered 6 to 18 months after the first dose. Travelers can be considered to be protected four weeks after receiving the initial vaccine dose. Individuals who will travel to intermediate or high risk areas less than 4 weeks after the initial dose of vaccine should also be given IG (0.02 ml/kg of body weight). The vaccine series must be completed for long-term protection. Estimates derived from modeling techniques suggest that vaccine may provide protective antibody against hepatitis A for at least 20 years.
Travelers who are allergic to a vaccine component or otherwise elect not to receive vaccine should receive a single dose of IG (0.02 ml/kg of body weight) if travel is for less than 3 months. For prolonged travel or residence in developing countries, a higher dosage of IG should be used (0.06 ml/kg of body weight) and should be repeated every 5 months.
Prevaccination testing:
Prevaccination testing is not indicated for children because of their expected low prevalence of
prior infection. For some adult travelers who are likely to have had hepatitis A in the past
(i.e., persons older than 40 years of age, persons born in parts of the world with intermediate or
high levels of hepatitis A, or persons with clotting disorders), screening for HAV antibodies
(anti-HAV) before travel may be useful to determine susceptibility and eliminate unnecessary
vaccination or IG prophylaxis. Factors to consider before doing prevaccination testing include:
1) the cost of vaccination compared with the cost of serologic testing, including the cost of an
additional visit and 2) the likelihood that prevaccination testing will not interfere with completion
of the vaccine series.
Safety:
Immune globulin
Intramuscular IG prepared in the United States has an excellent safety profile. IG produced
in developing countries may not meet the standards for purity required in most developed
countries. Persons needing repeat doses in other countries should use products that meet U.S.
license requirements.
Hepatitis A Vaccine
Experience to date indicates that Hepatitis A vaccine has an excellent safety profile.
Approximately 50,000 persons have received HAVRIX® in clinical studies. No serious adverse
events have been observed which could be attributed definitively to hepatitis A vaccine. In
combined clinical trials, 16,252 doses of VAQTA® were given to 9,191 healthy children,
adolescents and adults. No serious vaccine-related adverse experiences were observed during
clinical trials. For both vaccines, the most common side effects are mild problems that usually
disappear within 1 to 2 days. These may include soreness or swelling at the site of injection,
headache, tiredness and/or loss of appetite. As with any medication, there are very small risks that
serious problems such as severe allergic reaction and even death could occur after getting the
vaccine. Most people who have received hepatitis A vaccine have no problems from it.
Contraindications:
Immune globulin
Pregnancy is not a contraindication to the use of IG. Serious adverse events from IG are rare.
Hepatitis A vaccine
Hepatitis A vaccine should not be administered to persons with a history of hypersensitivity
reactions to components in the vaccine (i.e., alum or the preservative 2-phenoxyethanol).
Vaccination of an immune person is not contraindicated and does not increase the risk of adverse
events. Because the vaccine is inactivated, no special precautions need to be taken in vaccination
of immunocompromised persons. The theoretical risk to the developing fetus is expected
to be low when vaccine is administered to pregnant women. No animal or human data exist from
which to determine the safety of hepatitis A vaccination during pregnancy. The theoretical
risk of vaccination should be weighed against the risk of hepatitis A in women who may be at high
risk of exposure to HAV.
FIGURE 1. Endemicity patterns (low, intermediate and high) of hepatitis A virus infection worldwide. (Note: this map generalizes available data and patterns may vary within countries)